ERX-315 introduced at SABCS 2023
San Antonio, TX. December 7, 2023: Etira is pleased to present the data with its lead compound ERX-315 that cause catastrophic levels of ER stress, resulting in breast cancer cell death, and that can successfully function against multiple forms of endocrine therapy resistant breast cancer, including those driven by mutant estrogen receptor. Preclinical research, GMP manufacturing, formulation and IND-enabling investigations are all being completed in time for the phase I clinical trial to begin in Q2 2024.
Suryavathi Viswanadhapalli, Karla Parra, Tae-Kyung Lee, Rahul Gopalam, Kara Kassees, Tanner Reese, Gaurav Sharma, Xihui Liu, ChiaYuan Chen, Carlos Roggero, Yang Xue, Liping Chen, Sautam Bhattacharya, Uday P. Pratap, Russell Hayward, Sharron Gargosky, Jung-Mo Ahn, Ganesh V. Raj, Ratna K. Vadlamudi
Background: Estrogen receptor alpha (ERα) mutations are common (30-40%) in metastatic endocrine therapy-resistant breast cancers (ETR-BC), enable resistance to endocrine therapies and are the molecular drivers of ETR-BC. We had previously shown that an oligobenzamide, ERX-41, could enhance endoplasmic reticulum stress in ETR-BCs driven by mutant (MT) ERα, resulting in cancer cell death in vitro and in vivo. To enable clinical translation of ERX-41, we performed lead optimization, followed by preclinical and IND-enabling studies.
Methods: Over 2000 oligo-benzamides were designed, and tested in multiple BC models including those that express WT-ERα (MCF7, and ZR75) and BC models with acquired resistance (MCF7-TamR, and MCF7-LTLT) and engineered models that express MT-ERα (MCF7-ERα-D538G, MCF7-ERα-Y537S, ZR75-ERα-D538G, ZR75-ERα-Y537S). Mechanistic validation studies were performed using CRISPR LIPA mutants, RT-qPCR and Western blotting. Explants, organoids, cell line-(CDX) and patient-derived (PDX) xenografts were used to test the ex vivo and in vivo effectiveness of lead compound as a monotherapy and in combination with abemaciclib.
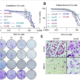
Results: Testing of >2000 synthesized oligo-benzamides identified a lead compound, ERX-315, that had broad and potent activity (IC50 between 20-100 nM) against both WT and MT (mutant) ERα-driven BC cells in in vitro assays. CRISPR KO of LIPA (which encodes lysosomal acid lipase (LAL) in BC cells abrogated cytotoxic response to ERX-315, validating LIPA as the molecular target of ERX-315. Ultrastructural and molecular studies confirmed that ERX-315 induces significant endoplasmic reticulum stress, leading to a shutdown of de novo protein synthesis and apoptotic cell death in BC cells. Importantly, ERX-315 does not induce endoplasmic reticulum stress or cell death in normal cells and is non-toxic in animal models. We have shown that this capacity of ERX-315 to induce endoplasmic reticulum stress is unique among drugs targeting ERα, including selective ERα modulators and degraders, such as GDC-0180, AZD-9496 and fulvestrant. ERX-315 has potent anti-proliferative activity against MT-ERα-driven BC, as spheroids in vitro, patient derived explants (PDEs) ex vivo and cell line derived (CDX) and patient-derived (PDX) xenografts in vivo. The combination of ERX-315 and CDK4/6 inhibitor abemaciclib was synergistic in decreasing the proliferation of both endocrine therapy-sensitive and therapy-resistant BC cells, in vitro and in vivo models. We are currently optimizing ERX-315 synthesis using GMP processes for kilogram scale production as well as approved methods for pharmacokinetic studies. We created formulations for both intravenous and oral administration that have excellent pharmacokinetic properties and have demonsratedin vivo utility of the formulated ERX-315 against CDX and PDX tumors. Toxicity studies in dogs and rodents have demonstrated >8-fold therapeutic to toxicity window.
Conclusions: We identified a lead compound ERX-315, that represents a novel class of agents that cause catastrophic levels of ER stress, resulting in BC cell death, and that can successfully function against multiple forms of ETR-BC, including those driven by MT-ERα. Preclinical research, GMP manufacturing, formulation and IND-enabling investigations are all being completed in time for the phase I clinical trial to begin in Q1 2024.